The BioRMB™ platform is especially well-suited for sensitive modalities—such as gene therapy products, fusion proteins, DNA, and mRNA—providing much higher recoveries and better product quality
Applications
mRNA
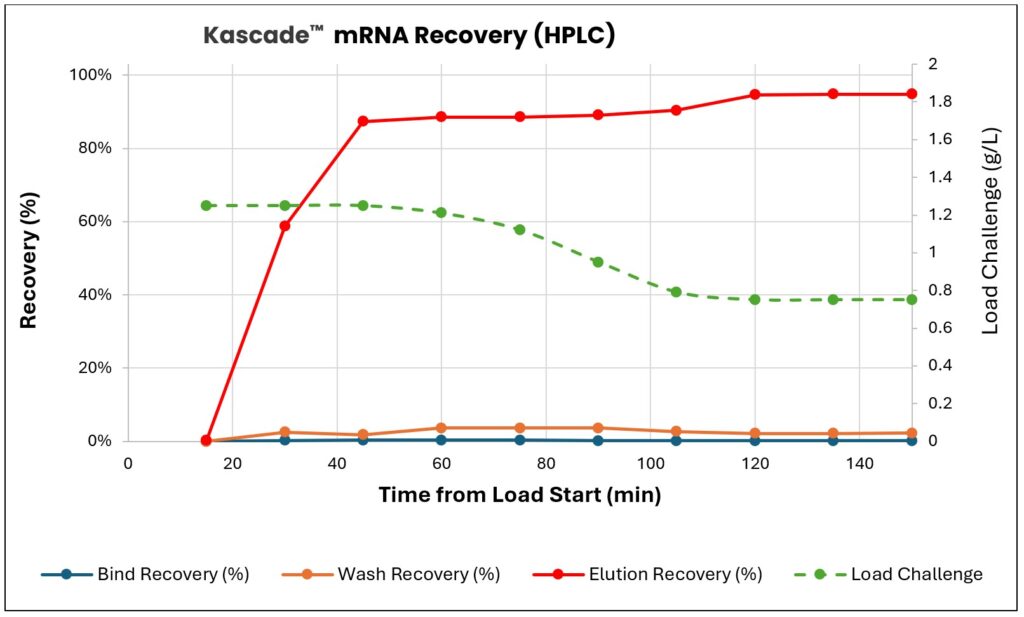
Messenger RNA and other nucleic acid therapeutic formats offer quicker development timelines particularly for vaccines. This new modality benefits from BioRMB with improved yields and higher productivity, enabling rapid response to critical public health emergencies. By coupling continuous In Vitro Transcription to the Kascade system customers can intensify their process for efficiency.
AAV
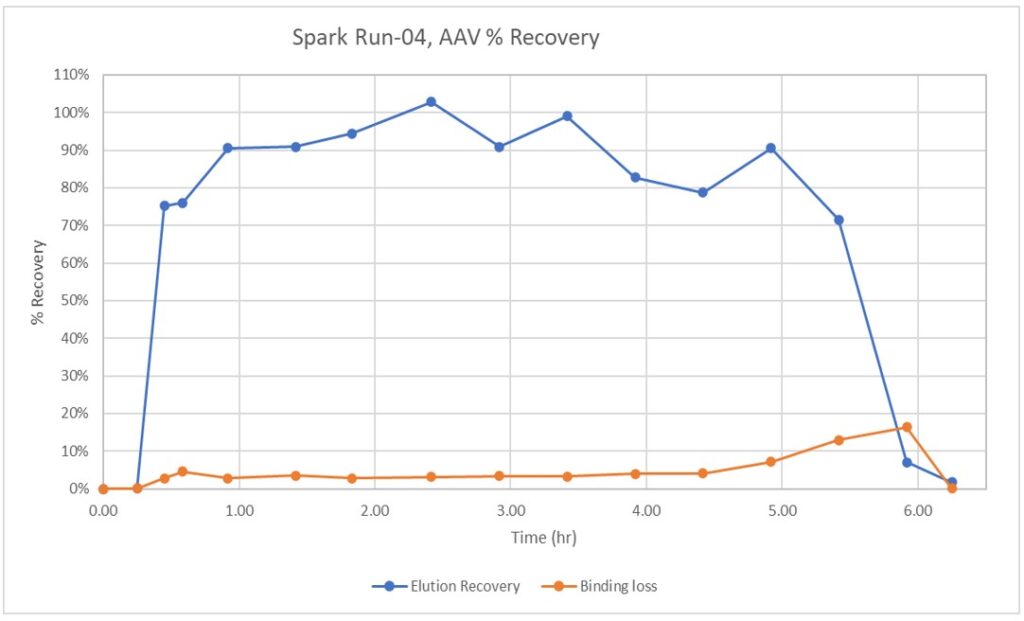
Adeno Associated Virus has emerged as the leading vector for gene therapy but it’s cell culture titers and purification yields still limit it’s applications and increase it’s cost of goods. BioRMB™ technology can provide improved recoveries for the capture unit operation while improving empty/full/partial separations in Anion Exchange polish chromatography. The flexibility of the Kascade™ system even allows for connection of these two steps, with dynamic product quality control through on-line monitoring.
Lentivirus
The purification of Lentivirus is difficult path to follow because of it’s fragility and primary contaminant profile. Purification by BioRMB™ is inherently quick, with on resin time measured in minutes versus hours and with on-line elution neutralization part of the Kascade™ system it can help retain infectivity of your viral particles.
mAbs
As the largest recombinant therapeutic class, monoclonal antibody processes can be improved through BioRMB™ by increasing productivity, lowering costs and intensifying processing. The continuous elution of the Kascade system allows for minimized surge vessels and easy coupling to flow through applications post Protein A capture.
Plasmids
Plasmids represent a critical raw material for both mRNA and AAV production processes. The production through fermentation, Anion Exchange capture chromatography and Hydrophobic Interaction polish chromatography creates a platform where endotoxin and plasmid purity can be improved through the implementation of BioRMB™. In addition, the efficient resin usage creates a lower overall cost to the basic platform.