Kascade™
System Specifications

Kascade™ General Description
The Kascade™ BioRMB™ System enables advanced process development, preclinical and GMP manufacturing of your continuous purification processes. With flow rates of X to Y mls/min and a resin utilization of A to B mls, the KASCADE system can be set up to execute complex online DOE studies, purify preclinical amounts of material or by using the available sterile single use flow path, produce GMP grade clinical material.
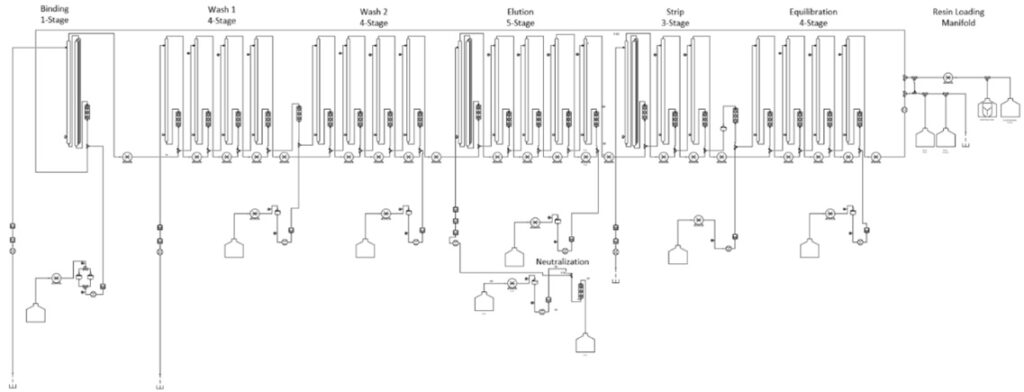
The Kascade™ is designed with 6 operating zones mirroring a typical purification method, Load, Wash 1, Wash 2, Elution, Strip and Equilibrate. Each operating zone is physically represented by an individual cassette and an additional static mixer in the Load and Elution zones. This modular approach allows for a fully enclosed sterile flow path with tool-less installation and commonality of parts.
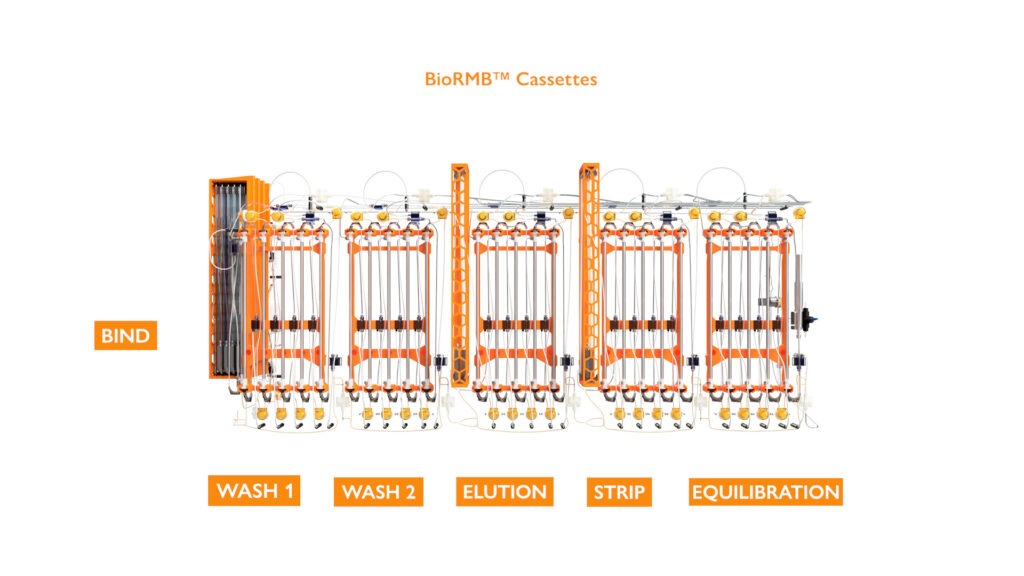
Kascade™ Operation
The simultaneous operation of these zones on the system produces a constant stream of purified product resulting in an elution plateau versus a traditional peak. The continuous processing enables an increased level of intensified manufacturing allowing for in-line capture from a continuous IVT, perfusion cell culture or fermentation system as well as in-line concentration and dilutions, even allowing for connection of two chromatographic unit operations in series.
The included proprietary Buffer Calculator and Experimental Design software allow for easy scale down modeling in 96 well plates and beaker/syringe systems. These models provide transferable parameters to the Kascade™ system to minimize confirmation runs. By mapping and controlling buffer interactions between chromatographic zones through the Gamma (Ƴ) value you can create precise conditions for loading, washing and elution.
Using proprietary mixers and maintaining laminar flow allows the Kascade™ system to operate continuously for days with a small volume of resin, driving efficient use of materials and lowering cost of goods. The optional gamma irradiated flow path also ensure sterile operation for long period production cycles.
Dimensional Specifications
165.1 cm (65.0 in.)
Length
55.9 cm (22 in.)
Width
66.0 cm (26 in.)
Height
175 kg (385 lb.)*
*Weight (Estimated)
Fits on standard 6’ lab bench
Placement
Purification Specifications
Minimum Flowrate: 2 mL/min
Maximum Flowrate: 25 mL/min
Cycle Time: 15 – 40 minutes
Runtime: 4+ days
Resin Cycle Limit: 100+ cycles
Resin Required: 10 – 40 g
Operating Pressure: 0 – 15 psi (30 max.)
Batch Size: 0.1 – 30+ Liters
Product Monitoring: pH, Conductivity, 280nm UV
Filtration Compatibility: Hollow fiber membranes, ChromaTan Dean’s Vortex Separators
Sanitization: Single-use flowpath, CIP sanitization compatibility

